Class 12 Chemistry Chapter 1 Solid state textbook solutions
Maharashtra state Chemistry Textbook Solutions for Class 12 are very important and crusial that helps the students in understanding the complex topics and helps them in the preparation of class 12 board examination as well as verious compititive entrance examinations also. Studying the answers to the questions in the Chemistry textbook will check your understanding of a particular topic and helps you determine your strengths and weaknesses.
Class 12 chemistry textbook Solutions for Class 12, Chemistry Chapter 1 solid state maharashtra state board are provided here with simple step-by-step detailed explanations. These solutions for solid state are very popular among Class 12 students for chemistry chapter 1 Solid state Solutions come handy for quickly completing your homework and preparing for exams. All questions and answers from the chemistry textbook Solutions Book of Class 12 chemistry Chapter 1 are provided here for you for free. You will also love the experience on ybstudy class 12 Solutions. All chemistry textbook Solutions. Solutions for class 12, These chemistry textbook solutions are prepared by Chemistry experts and are 100% accurate.
1. Choose the most correct answer.
i. Molecular solids are
a. crystalline solids
b. amorphous solids
c. ionic solids
d. metallic solids
ii. Which of the follwong is n-type semiconductor?
a. Pure Si
b. Si doped with As
c. Si doped with Ga
d. Ge doped with In
iii. In Frenkel defect
a. electrical neutrality of the
substance is changed.
b. density of the substance is
changed.
c. both cation and anion are missing
d. overall electical neutrality is
preserved.
iv. In crystal lattice formed by bcc unit
cell the void volume is
a. 68 %
b. 74 %
c.32 %
d. 26 %
v. The coordination number of atoms in
bcc crystal lattice is
a. 2
b. 4
c. 6
d. 8
vi. Which of the following is not
correct?
a.Four spheres are involved in the
formation of tetrahedral void.
b. The centres of spheres in octahedral
voids are at the apices of a regular
tetrahedron.
c.If the number of atoms is N the
number of octahedral voids is 2N.
d. If the number of atoms is N/2, the
number of tetrahedral voids is N.
vii. A compound forms hcp structure.
Number of octahedral and tetrhedral
voids in 0.5 mole of substance is
respectively
a. 3.011×1023, 6.022×1023
b. 6.022×1023, 3.011×1023
c. 4.011×1023, 2.011×1023
d. 6.011×1023, 12.022×1023
vii. Pb has fcc structure with edge length
of unit cell 495 pm. Radius of Pb
atom is
a. 205 pm
b. 185 pm
c. 260 pm
d. 175 pm
Answer the following in one or two
sentences
i. What are the types of particles in each of the four main classes of crystalline solids ?
Answer : Classes of Crystalline Solids. Crystalline substances can be described by the types of particles in them and the types of chemical bonding that takes place between the particles.
There are four types of crystals:
(1) ionic,
(2) metallic,
(3) covalent network, and
(4) molecular.
ii. Which of the three types of packing
used by metals makes the most efficient use of space and which makes the least efficient use?
Answer : silver, copper and alluminium
iii. The following pictures show population of bands for materials having different electrical properties.
Classify them as insulator,
semiconductor or a metal.
Answer : first is conductor, second insulator, third was semiconductor.
iv. What is the unit cell?
Answer : The smallest repeating structural unit of a crystalline solid is called unit cell.
v. How does electrical conductivity of a semiconductor change with temperature? Why?
Answer : The electrical conductivity of semiconductors increases with increasing temperature because, with increase in temperature, number of electrons from the valence bond can jump to the conduction band in semiconductors.
vi. The picture represents bands of
MOs for Si. Label valence band,
conduction band and band gap.
Answer :
vii. A solid is hard, brittle and electrically nonconductor. Its melt conducts electricity. What type of solid is it?
Answer : Generally, ionic crystals form from a combination of Group 1 or 2 metals and Group 16 or 17 nonmetals or nonmetallic polyatomic ions. Ionic crystals are hard and brittle and have high melting points.
viii. Mention two properties that are
common to both hcp and ccp lattices.
Answer: A CCP arrangement has a total of 4 spheres per unit cell and an HCP arrangement has 8 spheres per unit cell. However, both configurations have a coordination number of 12. The packing efficiency is the fraction of volume in a crystal structure that is occupied by constituent particles, rather than empty space.
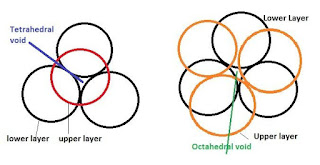
ix. Sketch a tetrahedral void.
Answer :
x. What are ferromagnetic substances?
Answer : Ferromagnetic materials are those materials which exhibit a spontaneous net magnetization at the atomic level, even in the absence of an external magnetic field.
3. Answer the following in brief.
i. What are valence band and
conduction band?
Answer : Conduction band : The highest energy band containing electrons is the conduction band. It is formed by interaction of the outermost energy levels of closely spaced atoms in solids. Conduction band may be partially occupied or vacant. Electrons in conduction band are
mobile and delocalized over the entire solid.
Valence band : The band having lower
energy than conduction band is the valence band. The electrons in valence band are not free to move because they are tightly bound to the respective nuclei.
ii. Distinguish between ionic solids and
molecular solids.
Answer :
- Ionic solids are solid compounds composed of oppositely charged ions held together by electrostatic attractions. A molecular solid is a type of solid in which molecules are held together by van der Waals forces rather than by ionic or covalent bonds.
- Ionic solids have ionic bonds. Molecular solids have mainly Van der Waal forces, and there can be hydrogen bonds, dipole-dipole interactions, London forces, etc. as well.
- Ionic solids have strong bonds. Molecular solids have weak bonds.
- Ionic solids are hard and brittle. Molecular solids are relatively soft.
iii. Calculate the number of atoms in fcc
unit cell.
Answer : Total number of atoms in one fcc unit cell =1+3=4. The number of atoms contained in one body-centred cubic unit cell of monoatomic substance is 2. Total number of atoms in one fcc unit cell =1+1=2.
iv. How are the spheres arranged in first layer of simple cubic close-packed structures? How are the successive layers of spheres placed above this layer?
Answer : In the first method, each successive layer of spheres covers gaps in the previous layer. Three neighboring spheres in the first layer will form a hollow space where they meet. Spheres in one layer align to fit in the hollows formed in the previous layer. The third layer aligns directly above the first layer.
v. Calculate the packing efficiency of
metal crystal that has simple cubic structure.
Answer : The efficiency of packing in case of simple cubic unit cell is given below: A simple cubic unit cell contains one atom per unit cell. Also, a=2r, where a is the edge length and r is the radius of atom. Total volume of unit cell =a3
vi. What are paramagnetic substances?
Give examples.
Answer : Paramagnetism refers to a property of certain materials that are weakly attracted to magnetic fields.Some of the examples of paramagnetic materials include iron oxide, oxygen, titanium, aluminium, transition metal complexes, etc
vii. What are the consequences of
Schottky defect?
Answer : Presence of large number of Schottky defect lowers the density of the crystal. When Frenkel defect alone is present, there is no decrease in density. The closeness of the charge brought about by Frenkel defect tends to increase the dielectric constant of the crystal.
viii. Cesium chloride crystallizes in cubic
unit cell with Cl ions at the corners and a Cs⊕ ion in the centre of the
cube. How many CsCl molecules are
there in the unit cell?
Answer : There are 2 atoms per unit cell as CsCl crystallises in body centered cubic crystalline structure.
ix. Cu crystallizes in fcc unit cell with edge length of 495 pm. What is the radius of Cu atom?
Answer :
FCC unit cell :
a = (2√2)*r
r = a/(2√2)
r = 495/2√2
= 495/2*1.41 (√2 = 1.41)
= 495/2.82
= 175.5 pm
x. Obtain the relationship between density of a substance and the edge
length of unit cell.
Answer :
i. If edge length of cubic unit cell is a, the
volume of unit cell is a3.
ii. Suppose that mass of one particle is m and that there are n particles per unit cell.
Mass of unit cell = m × n (1.1)
iii. The density of unit cell (ρ), which is same as density of the sub-substance is given by
ρ = mass of unit cell/volume of unit cell =
m×n/a3 = density of substance (1.2)
iv. Molar mass (M) of the substance is given
by
M = mass of one particle × number of particles
per mole
= m×NA (NA is Avogadro number)
Therefore, m= M/NA (1.3)
v. Combining Eq. (1.1) and (1.3), gives
ρ = n M/a3 NA. (1.4)
By knowing any four parameters of Eq. (1.4), the fifth can be calculated.